Stretched Acrylamide Gels
The weak alignment of biomolecules is achieved using stretched acrylamide gels for the measurement of residual dipolar couplings.
Ingredients
- Gel Stock solution for negatively charged gels (4.2% AMPS). Store at 4oC.
- 2.0ml of 40% acrylamide
- 1.14ml of 2% bis-acrylamide
- 0.65ml of 40% AMPS (2g of 2-acrylamido-2-methylpropane sulfonic acid to 5ml of 150mM Tris pH 8)
- 2.28ml of 150mM Tris pH 8
- 150mM Tris pH 8, 10ml.
Protocol
- Prepare fresh APS and TEMED solutions (store at 4C) :
- APS (Ammonium persulfate) : dissolve 10mg of APS in 300ul of 150mM Tris pH 8.
- TEMED (tetramethylethylenediamine) : dissolve 3.3ul of 94% TEMED liquid into 96.7ul of 150mM Tris pH 8
- A 280ul, 5.2% gel :
- 83ul of gel stock (Final concentrations (FC) : acrylamide = 3.9%, bis = 0.11%, AMPS = 1.3%)
- 182ul of 150mM Tris pH 8
- 7.5ul of 3.3% TEMED (FC = 0.088%)
- 7.5ul of 3.3% ammonium persulfate (FC = 0.088%)
3.Pour into mold (5.4mm) and cover with butyl alcohol. Allow to polymerize for a couple of hours.
-
Soak the polymerized gel overnight (or longer) in target buffer solution (ex : 100mM Tris pH 7.4).
-
Soak overnight (or longer) in H2O.
-
Dry the acrylamide gel. It is easiest to do this in a petri dish. Use a kim wipe to remove the liquid surrounding the gel, and stand the gel on its head, using two plastic inoculation loops. Place the gel in a 37C incubator and rotate the gel head-to-tail once or twice at 6-hour intervals. This is to allow even drying.
-
When the gel has dried (overnight), carefully remove it by scrapping it out with a razor blade.
-
Drop the dried acrylamide pellet into 280ul of degassed protein solution. Degassing the protein solution for 5-10 minutes with house vacuum to removes dissolved oxygen, which can form bubbles in the gel.
9 Soak the pellet for 2-4 days with the protein solution–longer soak times are necessary for large molecular-weight proteins and complexes. You may need to copolymerize the gel, if this is the case.
- See the gel loading aparatus in Chou, et al. for instructions on loading the gel into an open-ended NMR tube. SAG tubes and gel-loading apparatuses can be purchased from New Era.
Results
The cast gel should produce a 2H2O residual quadrupolar coupling (RQC) of about 0.8-2.0 Hz. Here is a sample 2H2O spectrum for a SAG sample at 750 MHz.
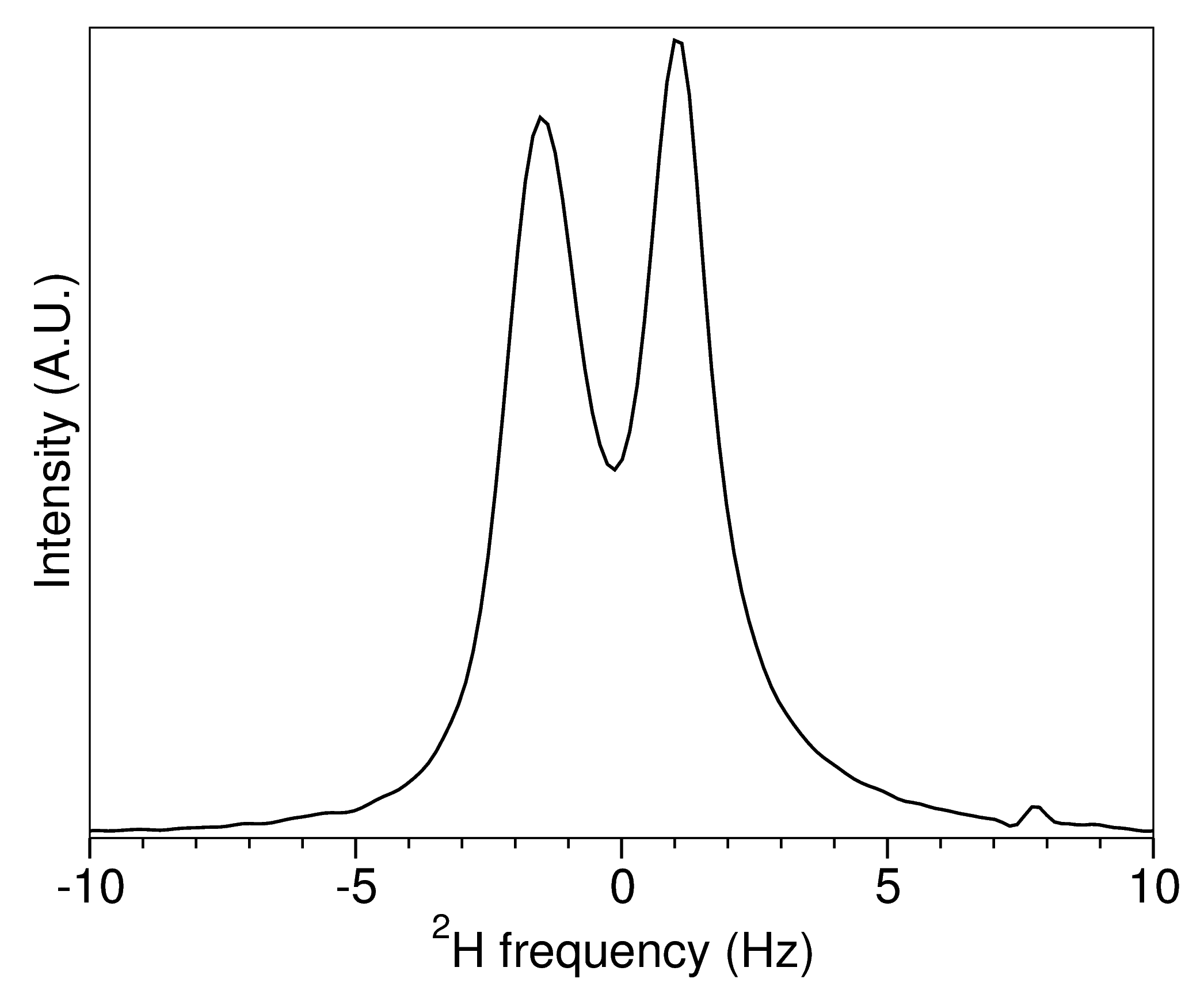
The quadrupolar splitting is not symmetric because the degree of compression and alignment throughout the sample is not homogeneous.
The final degree of alignment for the protein will be different. For example, with the hemagglutinin fusion peptide domain in DPC micelles, the 1H-15N Da was about 10 Hz for the sample that produced the 2H spectrum above.
References
-
Tycko R, Blanco FJ & Ishii Y (2000). Alignment of Biopolymers in Strained Gels: A New Way To Create Detectable Dipole−Dipole Couplings in High-Resolution Biomolecular NMR.J Am Chem Soc, 122 (38), 9340-9341.
-
Chou JJ, Gaemers S, Howder B, Louis J & Bax A (2001). A simple apparatus for generating stretched polyacrylamide gels, yielding uniform alignment of proteins and detergent micelles. J Biomol NMR, 21(4), 377-382.
-
Cierpicki T & Bushweller JH (2004). Charged Gels as Orienting Media for Measurement of Residual Dipolar Couplings in Soluble and Integral Membrane Proteins. J Am Chem Soc,126 (49), 16259-16266.
Revision Log
- 20150322 - Added the results section and a sample 2H spectrum.
- 20130525 - Initial posting

